DAN discusses the dangers of oxygen toxicity when using nitrox as a breathing gas.
By Dr. E.D. Thalmann, DAN Assistant Medical Director; Captain, Medical Corps, U.S. Navy (retired)
It’s a fact: we need oxygen to live. It’s because of the way our cells use oxygen that we are able to breathe, exercise, and even think. In each of our cells, structures called mitochondria take the oxygen which diffuses in from our blood, disassemble it into its two component atoms (remember, oxygen – O2 – is composed of two oxygen atoms), and then hook some available hydrogen nuclei to them to form water.
The process releases energy, which is used for all functions of life. The problem is that in disassembling the oxygen molecule, it involves a step in which an extra electron is hooked on. This forms an intermediate called a superoxide anion, and this is a bad actor. It is highly reactive, and it will make mincemeat out of most other molecules it comes in contact with.
These anions are like coals in a furnace: as long as they are contained, we get lots of safe chemical energy; if they get out we get a great deal of damage. The mitochondria are designed to contain these superoxide anions, but just in case some get loose, there are a host of protective chemical reactions designed to sop them up and prevent them from doing any damage.
Besides producing excessive amounts of the superoxide anion, elevated tissue oxygen levels also affect a variety of other biochemical reactions which may affect oxygen toxicity in ways that are only beginning to be understood. Tissue-protective mechanisms and biochemical reactions are tuned to life in an atmosphere containing 21 percent oxygen, or 0.21 atmospheres absolute (ata) oxygen partial pressure. (See sidebar: “Remember Partial Pressure?”, page 34.) As the partial pressure increases above this comfortable 0.21 ata, protective mechanisms are slowly overwhelmed and biochemical reactions are affected. This may eventually result in “oxtox,” or oxygen toxicity.
Oxtox – What Is It?
Oxygen toxicity is a time duration phenomenon: that is, both time and partial pressure play a role. If an oxygen partial pressure of 2 ata is breathed for a few minutes, there would probably not be any problem. But, breathing it for an hour, might cause problems. This is why oxygen exposure limits are given as partial pressure/time limits. As the partial pressure gets higher, the recommended exposure time gets shorter.
What kind of problems might breathing a high oxygen partial pressure cause? It is the lungs and the brain which are the target organs of major concern in diving oxygen toxicity. Oxygen toxicity in the lungs (pulmonary oxygen toxicity) is like getting a bad case of the flu, but it will rarely cause permanent damage. The most common situation in which pulmonary oxygen toxicity might occur is during very long recompression treatments.
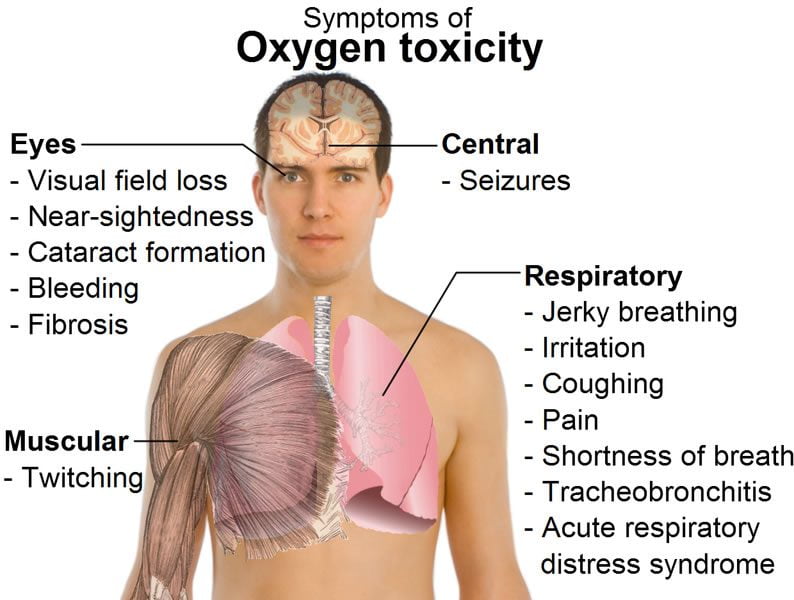
Oxygen toxicity of the brain, commonly referred to as central nervous system (CNS) oxygen toxicity, is different. It can occur during actual diving, and when it does, it can ruin your day – and possibly more. Some symptoms of CNS oxygen toxicity include flashing lights in front of the eyes, tunnel vision, loud ringing or roaring in the ear (tinnitus), confusion, lethargy, a feeling of nausea or vertigo, areas of numbness or tingling, and muscular twitching, especially of the lips.
These CNS symptoms are inconvenient, and a warning to change to a breathing gas with a lower oxygen partial pressure as soon as possible, but do not put the diver at risk of injury at this point. The big daddy of CNS symptoms does, however. It is the full-blown grand mal convulsion. During a convulsion, a diver will thrash about, perhaps bang his head into something hard, or if underwater, may lose his mouthpiece. The result can be trauma or drowning.
The good news is that convulsions are rare; the bad news is that all the inconvenient CNS symptoms noted above do not always provide warning of an impending convulsion. In some cases, a convulsion may occur without any warning at all. One more piece of good news: the convulsion in and of itself is not harmful, so if you don’t crack your head or drown, you should have no permanent damage. By now you’re probably asking where these dire descriptions are leading.
To a better understanding, we hope, of diving on nitrox. As air-breathing sport divers need to know about decompression sickness (DCS), divers using high oxygen in nitrogen mixtures (nitrox) need to know about oxygen toxicity. (To read more about nitrox, see Alert Diver, January/February 1996, p.32.)
Both decompression sickness and oxygen toxicity are rare occurrences; they can be made rarer with good diving practices. With DCS, it’s using your table or computer conservatively and keeping the ascent rate down. With oxtox, it’s paying attention to the partial pressure and the amount of exposure time.
The main thing we’re discussing here is CNS oxygen toxicity, because this is the most dangerous kind. Lung oxygen toxicity is unlikely to be a problem for recreational divers, so it will be mentioned only in passing.
Remember Partial Pressure?
The partial pressure of a gas is a measure of the number of molecules in a given volume – the molecular concentration. The physiological effects of a gas are due mainly to its partial pressure, no matter what the total pressure is.
If a gas has only one component, say 100-percent oxygen, the partial pressure and the pressure are the same. If there is a gas mix, then the partial pressure is the gas fraction times the total pressure. A 50 percent oxygen-in-nitrogen mix has an oxygen partial pressure (pO2) of 1.0 atmosphere absolute (ata) at a depth of 33 feet / 10 meters where the total pressure is 2 ata.
At this depth the 50 percent oxygen would have the same physiological effect as 100 percent oxygen at the surface. Breathing a 100 percent oxygen mix at a depth of 33 feet / 10 meters (2 ata total pressure) would be equivalent to breathing the 50 percent mix at 132 feet / 40 meters (4 ata total pressure).
Royal Navy Studies
The grand old man of CNS oxygen toxicity is Professor Kenneth Donald, who cut his teeth on the problem during World War II in Great Britain. (Want to know more? Read Reference 1, page 40.) At that time the Royal Navy was under pressure to develop the technology used by the Italians to severely damage the battleships HMS Queen Elizabeth and HMS Valiant in the harbor of the port city of Alexandria, Egypt, in 1941.
Italian divers wearing 100 percent oxygen rebreathers, drove a torpedo close into a ship. While submerged to avoid detection, they detached its warhead under the ship’s hull, and beat a hasty retreat after a timer was set.
The Royal Navy soon began developing its own band of underwater divers called “Charioteers” to carry out similar missions. Dr. Donald was assigned as a Surgeon Lieutenant to provide medical care during training of the divers using the British 100 percent oxygen rebreathers. The accepted safe limits for breathing 100 percent oxygen at the time (2 hours at 50 feet / 15 meters, 30 minutes at 90 feet / 27 meters) produced enough convulsions that the British Admiralty decided some sort of studies were needed to define the scope of the problem and, hopefully, find a solution.
About to be transferred to the Shetland Islands, Dr. Donald had a change of fortune and proceeded instead to a facility just outside of London, where he found himself heading up a major research effort to get a handle on the problem of CNS oxygen toxicity.
Royal Navy Discoveries
Over the next three years, Dr. Donald’s team conducted literally hundreds of exposures on human volunteers (remember, there was a war on). This series of studies formed the basis of what we know about CNS oxygen toxicity, namely:
- There is a large individual variation in susceptibility and time of onset to symptoms. This is what is referred to as “oxygen tolerance.”
- Compared to dry exposures, immersion decreases oxygen tolerance a great deal, decreasing exposure times up to a factor of four or five.
- Exercise decreases oxygen tolerance a lot, compared to rest.
- Diving in very cold (<49°F / 9°C) or very warm (>88°F / 31°C) water seems to decrease oxygen tolerance.
The goal of the research was to develop a set of oxygen exposure limits – that is, a table that indicated how long a diver could safely breathe 100 percent oxygen at various depths.
The main obstacle toward developing a good set of exposure limits was the large individual variation in oxygen tolerance. Not only did the time of onset and severity of CNS symptoms vary considerably between divers, but in a given diver there was a large day-to-day variation. One stalwart individual made dives twice a week for over three months on exactly the same dive profile (70 feet / 21 meters, 65°F / 18°C, at rest, 100-percent oxygen) until signs of oxygen toxicity developed (again, a notable contribution to the war effort!). His symptom onset time was random and ranged from seven minutes to 148 minutes!
As a result of these studies, the Royal Navy considered it unsafe to breathe 100 percent oxygen below a depth of 25 feet / 7.6 meters (an oxygen partial pressure of 1.76 ata). In fact 25 feet / 7.6 meters was the shallowest depth tested. No particular time limit was given for this exposure, but the longest time tested was two hours. The carbon dioxide absorbent canisters of the diving rigs of the day rarely lasted more than 90 minutes.
The Royal Navy made deeper dives by using nitrogen-oxygen mixtures in the newly developed semi-closed circuit rebreathers. This was the beginning of so-called “mixed-gas diving,” where the breathing gas is mixed from oxygen and nitrogen rather than simply being compressed from atmospheric air.
U.S. Navy Studies
In the 1950s, Dr. E.H. Lanphier, then a Lieutenant in the U.S. Navy Medical Corps, undertook a series of studies at the Navy Experimental Diving Unit (NEDU), located at that time in Washington, D.C., to investigate whether oxygen exposure limits could be developed for 100 percent oxygen dives deeper than 25 feet / 7.6 meters. Table 1 (below) shows the limits that he recommended. The 100 percent oxygen exposure limits in Table 1 remained in use up to 1970 and with only slight modifications were used through 1991 when they were again changed.
Dr. Lanphier was also charged with investigating how these limits should be applied to the oxygen partial pressures encountered in mixed-gas nitrox diving. During nitrox diving, oxygen partial pressures similar to those used in 100 percent oxygen diving may be encountered, but since nitrogen has been added, these partial pressures are reached at a greater depth and, therefore, at a greater breathing gas density.
U.S. Findings
From his studies, Dr. Lanphier concluded that the increased gas density encountered during mixed-gas nitrox diving required the exposure times at a given oxygen partial pressure to be shorter than for 100 percent oxygen rebreathers, which can be used only at shallow depths, and which result in a lower gas density. The reason for this decreased tolerance during nitrox diving was thought to be due to decreased carbon dioxide elimination at the greater depths, resulting in higher blood carbon dioxide levels. This would make the diver more sensitive to oxygen toxicity.
These U.S. Navy nitrox mixed-gas nitrogen-oxygen exposure limits are shown in Table 2 (page 36). Notice that compared to those for 100 percent oxygen breathing in Table 1, these are quite a bit shorter for the same partial pressure. With the advent of closed-circuit oxygen rebreathers, the U.S. Navy no longer uses nitrox scuba and no longer publishes nitrox exposure limits in their official diving manual.
The Conflict – and Some Good Advice
The British disagreed with Dr. Lanphier’s findings, and the Royal Navy set exposure limits for nitrox diving that were no different than for 100 percent oxygen diving. This area remains controversial – Dr. Donald’s case for keeping the exposure limits the same for both 100 percent oxygen and nitrox diving has weaknesses and should not be accepted as proven.
Dr. Lanphier’s work is certainly compelling enough that divers should be very cautious before extrapolating oxygen exposure limits based on 100 percent oxygen rebreathing directly to nitrox diving at higher gas densities. Ideally, nitrox limits should be tested at the maximum gas density anticipated for their use.
CO2 Retention
Why would carbon dioxide (CO2) retention become a problem at increased gas densities? There have been many studies showing that as depth increases while breathing air, the high oxygen and increased gas density will normally slow the rate at which we breathe and thereby the rate at which we eliminate carbon dioxide. This will raise the blood levels of carbon dioxide. On top of this, however, is the fact that, because of individual variations, not all divers will slow their breathing in the same amounts.
Dr. Lanphier investigated the problem of divers who tended to breathe more slowly during diving than would normally be expected – so-called “carbon dioxide retainers.” He felt that these individuals would be at an especially high risk of CNS oxygen toxicity when breathing high oxygen in nitrogen gas mixtures. Should a nitrox diver be concerned about whether he is a carbon dioxide retainer? Unfortunately, there is no good test to reliably identify carbon dioxide retainers. The best strategy at present is to use conservative oxygen exposure limits.
More U.S. Studies – Oxygen Exposure Limits
In the late 1970s and early ?s, the Navy Experimental Diving Unit (NEDU) – now moved to Panama City, Fla.- conducted a series of studies to look at longer exposure times breathing 100 percent oxygen at shallow depths while exercising at levels typically encountered by combat swimmers while swimming long distances underwater. (Remember, exposure times developed using divers at rest may well cause problems for exercising divers, since exercise decreases oxygen tolerance.)
The conclusion of the study was that four-hour exposures at 25 feet / 7.6 meters (1.76 ata) had a low probability of causing CNS symptoms but were not without hazard since a convulsion was reported at this depth after 72 minutes of exercise. Because of this hazard, it was recommended that routine exposures be carried no deeper than 20 feet / 6.1 meters (1.6 ata) for up to four hours, with a single excursion between 21 and 40 feet / 6.4 and 12 meters for 15 minutes, or between 41 and 50 feet / 12 and 15 meters for five minutes.
Even this recommendation does not completely eliminate the possibility of a convulsion. One diver had a convulsion at 20 feet / 6.1 meters approximately 48 minutes after making a 15-minute excursion to 40 feet / 12 meters at the beginning of the dive. These studies had their share of oxygen convulsions and verified their unpredictability as observed by Dr. Donald some 40 years earlier. One feature of these convulsions that deserves mentioning is that they usually occurred with little or no warning.
With the advent of nitrox diving it is wise to consider these studies. Dr. Andrea Harabin, a scientist at the Naval Medical Research Institute (NMRI) in Bethesda, Md., analyzed the human oxygen exposures from the NEDU studies and used a mathematical model to predict the probability of CNS oxygen toxicity symptoms occurring. (See Reference 2, page 40 for details.)
When she considered all symptoms which resulted in the diver stopping his dive, she found that the model had a threshold at 1.3 ata; that is, the probability of a CNS symptom occurring at or below this level should be essentially zero.
Some of the CNS symptoms that caused dives to be halted could have been due to many other reasons besides oxygen toxicity and were classified as “Probable.” In contrast, with “Convulsions” and “Definite Symptoms” (see Table 3, page 37), there is usually no question that oxygen toxicity is the culprit. When Dr. Harabin considered just the convulsions and definite symptoms, she found the thresholds to be 1.7 ata. This analysis again reflects the large degree of uncertainty inherent in these types of human exposures.
USN 100 Percent Oxygen Rebreather Exposure Limits (1954)
TABLE 1
| Normal Operations | |
| Depth (feet) 10 15 20 25 | Time (min) 240 120 90 65 |
| Exceptional Exposure Operations | |
| Depth (feet) 30 35 40 45 | Time (min) 45 34 25 15 |
USN Oxygen Exposure Limits for Nitrogen-Oxygen Mixed-Gas Diving (1956)
TABLE 2
| Normal Exposures | |
| Oxygen Partial Pressure (ata) 1.6 1.5 1.4 1.3 1.2 1.1 1.0 | Time (min) 30 40 50 60 80 120 240 |
| Exceptional Exposures | |
| Oxygen Partial Pressure (ata) 2.0 1.9 1.8 1.7 1.6 1.5 1.4 1.3 | Time (min) 30 40 50 60 80 120 240 |
What Oxygen Level Is Safe?
So, what levels of oxygen can be breathed safely? Currently, the U.S. Navy is using 1.3 ata as the maximum limit in its closed-circuit rebreathers – the more conservative threshold found by Dr. Harabin for exercising divers. Using these closed-circuit rigs, exposures exceeding eight hours are possible, and at the 1.3 ata level the chance of CNS oxygen toxicity should be very rare.
Very long exposures, however, may put the diver at risk for some lung toxicity symptoms. The National Oceanic and Atmospheric Administration (NOAA) takes a slightly more conservative approach, recommending 180 minutes at 1.3 ata for normal exposures and 240 minutes only for exceptional exposures (see Table 4). This additional conservatism, according to NOAA, “take(s) operational safety considerations into consideration and are sufficient in duration for anticipated NOAA dives.”
The NOAA limits shown in Table 4 are based on the results of the NEDU oxygen exposure limit studies done in the ?s, taking the increased gas densities encountered in nitrox diving into account. The “normal exposure limits” are longer than the nitrox limits proposed by Dr. Lanphier in Table 2 (page 36) but are quite a bit shorter than the 240 minutes, 1.6 ata exposure, currently allowed by the U.S. Navy for 100 percent oxygen diving. However, the “exceptional exposure limits” are virtually the same as originally recommended by Dr. Lanphier, showing that there has not been much change in opinion as to what is safe at these higher partial pressures.
PADI, the Professional Association of Diving Instructors, has proposed a limit of 1.4 ata for open-circuit nitrox scuba diving. Because open-circuit scuba diving would not expose divers to this level continuously, in practice it should be as safe, or safer, than the 1.3 ata U.S. Navy limit for continuous exposures. (See sidebar “Continuous vs. Intermittent Exposures,” page 40.) In fact, the shallow exposure times in the 1.3- to 1.4-ata range are mainly to avoid lung oxygen toxicity; the likelihood of CNS toxicity at these levels is very low and probably not much different over this range.
Is it possible to breathe oxygen at a higher oxygen partial pressure (pO2)?
The answer is yes, but! Dr. Harabin’s analysis gave a threshold limit of 1.7 ata (23 feet / 7 meters) for an exercising diver when considering only “convulsions” and “definite” symptoms. This is uncomfortably close to the 25-foot / 7.6-meter (1.76 ata) depth where a convulsion was reported, so backing off to 20 feet / 6.1 meters(1.6 ata) gives a little more breathing room.
Currently the U.S. Navy would allow an exercising exposure at this partial pressure for up to four hours, but that assumes breathing 100 percent oxygen at 25 feet / 7.6 meters by trained combat swimmers. A depth excursion of only 5 feet / 1.5 meters would put the diver in an area where convulsions have been reported, and divers who tend to retain carbon dioxide during exercise may be at increased risk.
The NOAA limit for nitrox diving at 1.6 ata is 45 minutes for normal diving and 120 minutes for exceptional exposure diving. Again, some conservatism is built into these limits and consideration given to the fact that this partial pressure may be breathed at higher gas densities than would be encountered by the divers using 100 percent oxygen.
During a nitrox dive done at Duke University’s F.G. Hall Hypo/Hyperbaric Center at 100 feet / 30 meters, breathing 1.6 ata pO2 (oxygen partial pressure) during heavy exercise, a convulsion occurred after 40 minutes. Perhaps this would not have occurred had there been a lower level of exercise, but it does seem to indicate that the NOAA limit of 45 minutes for 1.6 ata nitrox diving is not overly conservative.
Breathing 100 percent oxygen during the 20-foot / 6.1-meter decompression stop is common practice, and at this depth, the partial pressure will be about 1.6 ata. At this shallow depth, under conditions of rest, the chance of CNS oxygen toxicity should be very low. But, like most things in life, this is not certain, as evidenced by a recently reported oxygen convulsion at 20 feet / 6.1 meters during decompression by a technical diver after completing a dive on the Lusitania.
TABLE 3
Symptoms of CNS Oxygen Toxicity Encountered in NEDU Studies
Convulsions: the most serious symptom and the one to avoid at all cost.
Definite: muscle twitching, tinnitus (ringing in the ears), blurred or tunnel vision, disorientation, aphasia (inability to express oneself by speaking), nystagmus (rapid side-to-side motions of the eye), or incoordination.
Probable: more equivocal signs which could be due to oxygen toxicity as well as other causes: light headdress apprehension, dysphoria (“just didn’t feel right”), lethargy, and transient nausea.
Recommendations
One thing you should be impressed with by now is that oxygen toxicity is fickle; convulsions have occurred at shallow depths under conditions where most experts would not have expected them to occur.
So, as an air sport diver, how should you view nitrox diving? The answer is: carefully.
Experts rationalizing why particular oxygen exposure limits do or do not cause oxygen toxicity are like investment analysts rationalizing movements in the stock market – everyone has a reason, but know one really knows why!
First, whenever a gas is breathed with an oxygen fraction above 21 percent, you should assume that oxygen toxicity is a possibility and have appropriate training. This not only means having a buddy clearly visible at all times but also knowing what action to take should oxygen toxicity occur. (See sidebar: “What do you do if oxygen toxicity or a convulsion happens?” )
Second, using equipment designed to compress high oxygen mixtures can be hazardous in itself and requires special training.
Third, what you get in your tank may not be what you expect. A method of analyzing the amount of oxygen in the tank independent of the filling station must be available.
Fourth, if you are attracted to rebreathers, remember that they are complex pieces of life-support gear, requiring much more care and feeding than the good old scuba regulator. If you get into rebreathers, expect to get hit with good-sized training and maintenance costs.
Finally, there is the matter of keeping the possibility of oxygen toxicity to a minimum.
Moving Ahead
For open-circuit scuba diving, consider the “green light” region any oxygen partial pressure of 1.4 ata or less (this is about 82 feet / 25 meters on a 40-percent oxygen mix.) As long as this level is never exceeded, other limitations of open-circuit scuba diving will limit the exposure time to lengths where CNS oxygen toxicity is unlikely to be encountered, even for exposures approaching four hours.
Proceeding With Caution
Between 1.4 ata and 1.6 ata (this is 99 feet / 30 meters on a 40-percent mix) is the “yellow light” region. The possibility of oxygen toxicity at 1.6 ata is low, but the margin of error is very slim compared to 1.4 ata. Individual variation, the likelihood of an unplanned depth excursion causing an increase in oxygen partial pressure, and the possibility of having to perform heavy exercise in an emergency put the possibility of oxygen toxicity at levels where caution should be exercised. Thus, levels of 1.5 to 1.6 ata should be reserved for conditions where the diver is completely at rest, such as during decompression. Again, as noted previously, the dive team must still be prepared for the possibility of an oxygen convulsion at these levels.
Stop!
Above 1.6 ata is the “red light” area. Just don’t do it. Yes, there is evidence that short exposures at higher levels of pO2 (oxygen partial pressure) are possible but so are convulsions. At these levels, oxygen exposure depth/time limits must be adhered to. Even mild exercise may put divers breathing high-density nitrox mixes at increased risk; and even open-circuit scuba divers can achieve durations likely to get them into trouble at these levels. Diving using these high partial pressures of oxygen should be left to the trained professionals who can weigh the risks and benefits and who have the necessary training and support structure in place, if an oxygen convulsion occurs.
Finally…
Nitrox diving may extend bottom times or decrease the possibility of decompression sickness, depending on how it’s used, but it adds to the risk of oxygen toxicity.
Decompression sickness rarely occurs in the water and is rarely life-threatening. When it happens underwater, however, life support is usually not an issue – instead, attention is focused on getting to a treatment chamber. If an oxygen convulsion occurs, it almost always occurs underwater, greatly complicating treatment. So while the probability of a convulsion may be low, the possibility of severe injury or death is high if it does occur. Taken together this makes it a risky occurrence, and each diver needs to consider that risk whenever nitrox is used. Experience and good training are essential. This is an area that requires team diving, with the whole team full trained in nitrox diving.
What do you do if oxygen toxicity or a convulsion happens?
Editor’s note: After reading the article on nitrox in the January/February 1996 Alert Diver, a DAN member asked what the recommended procedure was in the event of an underwater oxygen convulsion. An oxygen convulsion in the water is rare but potentially life-threatening. Like learning CPR, practicing the proper handling of an oxygen convulsion is maintaining a skill you hope you’ll never use. The organization with the most experience with 100 percent oxygen diving is the United States Navy. Its recommendations for managing oxygen toxicity is as follows:
According to the USN Dive Manual sections 14.9.1.1 and 14.9.1.2 the suggested procedure for dealing with seizures is:
Management of Nonconvulsive Symptoms.
The stricken diver should alert his dive buddy and make a controlled ascent to the surface. The victim’s life preserver should be inflated (if necessary) with the dive buddy watching him closely for progression of symptoms.
Management of Underwater Convulsion.
The following steps should be taken when treating a convulsing diver:
- Assume a position behind the convulsing diver. Release the victim’s weight belt unless he is wearing a drysuit, in which case the weight belt should be left in place to prevent the diver from assuming a face-down position on the surface.
- Leave the victim’s mouthpiece in his mouth. If it is not in his mouth, do not attempt to replace it; however, if time permits, ensure that the mouthpiece is switched to the surface position.
- Grasp the victim around his chest above the underwater breathing apparatus (UBA) or between the UBA and his body. If difficulty is encountered in gaining control of the victim in this manner, the rescuer should use the best method possible to obtain control. The UBA waist or neck strap may be grasped if necessary.
- Make a controlled ascent to the surface, maintaining a slight pressure on the diver’s chest to assist exhalation.(see commentary below)
- If additional buoyancy is required, activate the victim’s life jacket. The rescuer should not release his own weight belt or inflate his own life jacket.
- Upon reaching the surface, inflate the victim’s life jacket if not previously done
- Remove the victim’s mouthpiece and switch the valve to SURFACE to prevent the possibility of the rig flooding and weighing down the victim.
- Signal for emergency pick-up.
- Once the convulsion has subsided, open the victim’s airway by tilting his head back slightly.
- Ensure the victim is breathing. Mouth-to-mouth breathing may be initiated if necessary.
- If an upward excursion occurred during the actual convulsion, transport to the nearest chamber and have the victim evaluated by an individual trained to recognize and treat diving-related illness.
Deciding whether to ascend with a diver who is convulsing can be tricky. In section 8-2.4 of Volume 1 of the U.S. Navy diving manual it states:
“If a diver convulses, the UBA should be ventilated immediately with a gas of lower oxygen content, if possible. If depth control is possible and gas supply is secure (helmet or full face mask), the diver’s depth should be kept constant until the convulsion subsides. If an ascent must take place, it should be done as slowly as possible. If a diver surfaces unconscious because of an oxygen convulsion or to avoid drowning, the diver must be treated as if suffering from arterial gas embolism.”
Obviously, a full face mask is the best way to perform diving with high oxygen mixes because the diver can be kept at depth until the convulsion subsides. If the diver is breathing from a mouthpiece and it comes out of his mouth, there is no option but to surface the diver, since when the convulsion stops he will try to take a breath. Training and practice are the only ways to ensure that divers will know how to bring a convulsing diver to the surface, using a slow, controlled ascent, if that becomes necessary.
In the section on the management of underwater convulsions, the reference to switching the mouthpiece to the surface position would refer only to rebreathers where an open mouthpiece which inadvertently becomes submerged can flood the UBA.
Also, step g should be modified if the victim is breathing nitrox using open-circuit scuba. If someone is convulsing, you won’t be able to remove the mouthpiece; and this should never be done by force. Once the convulsion subsides, if the mouthpiece is secure (or if the diver is wearing a full face mask) and if the diver is still in the water and breathing, then leave everything in place until you can get the injured diver out of the water. If he is not breathing, then remove the mouthpiece once on the surface and begin rescue breathing.
The main goal while the injured diver is in the water is to keep him from drowning. Next is to ensure that his airway is open after the convulsion stops by keeping the neck extended.
Finally, be on the lookout for foreign bodies in the trachea. It is possible to bite off the parts of the mouthpiece between the teeth during a convulsion, which can find their way into the trachea, blocking the airway. In these cases, the injured diver will begin coughing as he returns to consciousness, or he may try to breathe but not get any air into his lungs. Here you need to institute the standard procedures taught in CPR classes for foreign body obstruction of the trachea.
Continuous vs. Intermittent Oxygen Exposures
Remember that CNS oxygen toxicity symptoms are a time-duration phenomenon. They will not suddenly occur the minute a particular partial pressure is exceeded – it takes time. As you can see from the exposure limits in the tables (Table 4), as the inspired oxygen partial pressure increases, the exposure time decreases.
The U.S. Navy limit of 1.3 ata for continuous exposures reflects their desire to keep the risk of CNS symptoms essentially zero, no matter how long the dive.
In nitrox diving, however, divers breathe from open-circuit scuba with a fixed fraction of oxygen in the breathing mix. PADI has chosen 1.4 ata as the maximum open-circuit scuba limit; the limitations placed on duration by open-circuit scuba will ensure that the likelihood of CNS oxygen toxicity is no greater than would be experienced by the U.S. Navy closed-circuit divers.
When using open-circuit scuba, the 1.4 ata maximum oxygen partial pressure is reached only at the maximum depth, and for the vast majority of recreation divers, the time spent at this maximum depth will be limited to times where CNS oxygen toxicity is unlikely to be encountered. At all shallower depths, the oxygen partial pressure will be lower, and the overall exposure during the entire dive is unlikely to have physiological effects significantly different than a continuous 1.3 ata exposure. Be careful when extending this analogy to higher partial pressures, however. Formulas are available for integrating the exposures at various depths to predict overall exposure times when looking only at lung oxygen toxicity. This concept does have some support research done at Dr. C.J. Lambertsen’s laboratory at the Institute of Environmental Medicine in Philadelphia, Pa.
The case for CNS oxygen toxicity is much more complicated. Research done at the Navy Experimental Diving Unit (NEDU) in 1986 specifically looked at how brief exposures to oxygen partial pressures of 2.0 ata or greater would impact the overall exposure time at 20 feet / 6.1 meters of sea water (fsw). The results were not clear, and it was obvious that no formula could be developed which would allow integration of oxygen exposures at various depths into a single indicator which would help the diver avoid CNS oxygen toxicity. The best that could be said is that a single 15-minute excursion to 40 fsw/12 msw, or for five minutes at 50 fsw/15 msw, probably had no significant effect. This formed the basis of the current U.S. Navy recommendations. No such research has yet been carried out for high oxygen nitrox diving, to my knowledge.
REFERENCES
Donald KM. Oxygen and the Diver. England: Images, 1993. Available through Best Publishing Co., Flagstaff, Ariz. (This reference also covers all of the NEDU studies mentioned and gives full citations for them.)
Harabin AL, Survanshi SS. A statistical analysis of recent Navy Experimental Diving Unit (NEDU) single-depth human exposures to 100-percent oxygen at pressure. Bethesda, M.D. Naval Medical Research Institute Report NMRI 93-59, 1993. Note: Both NEDU and NMRI Reports are available through: National Technical Information Service, 5385 Port Royal Road, Springfield VA 22161.
DAN (Divers Alert Network) is run by divers – for divers and provides superb advice, education and insurance for all those who dive – regardless ofthe complexity of their involvement in the sport.